UNDAY, SEPTEMBER 21, 2014
Skincare Discovery: Why the pH of Your Cleanser Matters
So, in an earlier Randomness post (click here if you are curious) I mentioned I had purchased the Su:m37 Miracle Rose Cleansing Stick, despite it's expensive and difficulty in purchasing it, because I was becoming more and more engrossed in what role pH can have in your skincare.
This topic is too huge to be addressed in a single blog post, so I will be doing an ongoing series that will address different aspects of it as I collect research and form my admittedly hoi polloi understanding of it.
I will warn you before I start that this rabbit hole has no end, and therefore I will be glossing over certain terms and topics as well as reducing some explanations down to such a simplistic, generalized form as to trigger fits in any chemist wandering by my blog. (If that happens, O Chemist, please drop me a line because I have a million questions for you!)
There's a lot of misinformation out there from popular sources like the beauty 'experts' on Oprah's website, who makes claims such as: "Cleansers and toners are alkaline—hand soap typically has a pH of around 9 or 10, for example—because alkaline molecules bind to dirt and accumulated oils you want to wash off." and "Bottom Line: A claim that a product is pH balanced is more marketing tool than useful information." I admit I used to think this way, until reading a post from Skinandtonics on the acid mantle, which blew my mind.
So, I started to research and test it for myself, and hence me sharing the fruits of my labour with you today.
Included in this post:
- What is pH, I need a refresher?
- The pH of your skin, and does it matter?
- The pH of your cleanser, and does it matter?
- The claim that alkaline pH = efficacy, is it true?
Before we get into the pH details of cleansers, let's do a quick overview of pH in case your pre-high school science recollection is as spotty as mine.
What is pH, I need a refresher?
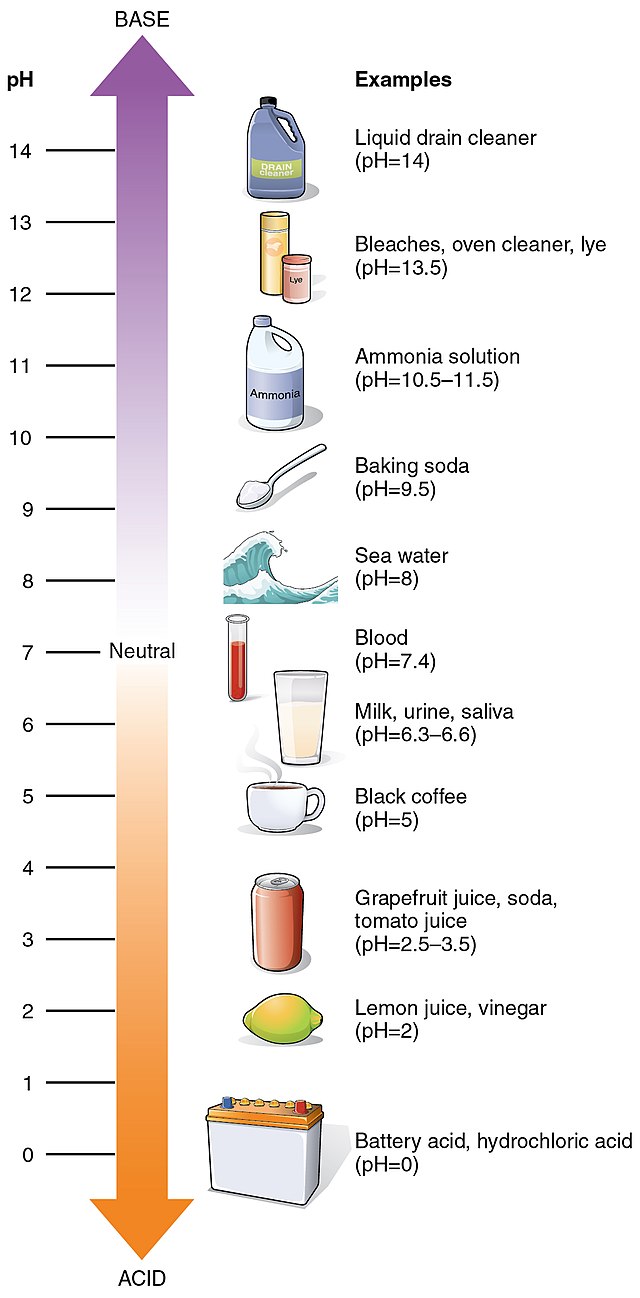 |
| source in img title |
pH is a chemistry measurement of the acidity vs alkalinity (or 'basicity', technically alkalinity is a combination of bases that- actually, you know what, just google it. Basicity may be the correct term but it's making my inner spellcheck sense freak out and we're going to be talking about cleansers and their alkalinity so I prefer to use that term) of an aqueous solution, aka a solution of water + something.
Technically, oils can also have a pH (and in fact the pH testing of oils is necessary in such petroleum products as biodiesel) but that's a whole other topic that I'm certainly not qualified to get into.
Back to pH: pH 7 is 'neutral', and anything below it is considered an 'acid', down to hydrochloric acid which has a pH of 0, and anything above pH 7 is considered a 'base' (or alkaline) all the way up to concentrated sodium hydroxide which has a pH of 14. There are substances that are considered beyond the 0-14 measurement scale known as 'superacids' and 'superbases' but since those aren't relevant to skincare, I'm movin' on here.
You can adjust the pH of something by adding a substance that is either more acidic or more alkaline, and they will react to one another. The final product will land somewhere between the original starting points. They may also produce other chemicals by doing so, so never try to DIY pH adjust at home, kids.
This means that anything you put on your skin that is higher or lower than it's natural pH will correspondingly raise or lower the skin's pH as well. This can either be bad (weakening your skin's defenses) or good (restoring its natural state). One of the most common 'good' uses is the use of AHA and BHA acids in skincare. They're popular for a reason! AHAs and BHAs are another day, though.
Before we go on, let me interject a plea: never use lemon juice on your skin. Ever. I know that pinterest and Michelle Phan and a whole bunch of DIY beauty gurus out there espouse the use of lemon juice as an at-home beauty treatment all the time, but please don't do it. Don't believe me? Google "lemon juice chemical burn" and have a picture of a bunny nearby for a visual chaser, because you're going to be mentally scarred afterward.
Even more important! Never use baking soda on your skin. EVER. We will go deep into why anything alkaline, or basic, on your skin is a terrible terrible idea.
Interestingly, the pH range of 'healthy' adult skin is actually acidic, not neutral, and this is where all this science magic comes into play. [source: Natural skin surface pH is on average below 5, which is beneficial for its resident flora.] In fact, healthy adult skin is between 4.2 and 5.6, and men have slightly more acidic skin than women. [source]
The pH of your skin, and does it matter?
Yes, it matters. Specifically, it matters a great deal when it comes to maintaining the health of your skin overall, but also specifically acne. Brace yerselves for some science! Note: I have replaced the original citations of secondary sources with links to the actual source to avoid you having to dig through the primary study's bibliography. You're welcome.
One of the articles I'm going to quote at length is The pH of the Skin Surface and Its Impact on the Barrier Function, which has some excellent information not only on what can change the pH of your skin but also what negative effects it has. All of the references in this section, and the following section, are from this source unless explicitly stated otherwise.
It liberally uses the term 'syndet' which is a 'synthetic detergent', a modern era invention that does not rely on the functions of traditional soap to uh .. clean things. Phew. I was about to get lost on the surfactant/hydrophobic and hydrophilic molecule train but I stopped myself just in time!
 |
| It was a very near thing. Chemistry is fascinating! Source in image. |
Healthy, acidic skin in a 'good' pH range does all sorts of wonderful things for you, like resisting bacteria, preventing your skin from losing water (called TEWL or transepidermal water loss), resisting disease, and a whole host of other sparkly things that you can read up on, if you're curious. Because this blog post is about cleansing, I'm going to focus on what cleansers do to skin's pH and fall out as a result.
The pH of your cleanser, and does it matter?
You might think that water, being pH neutral but more importantly the most common thing used in cleansing your skin, is beneficial to your skin or at least, harmless as far as pH goes. However, that's not the case:
Even rinsing the skin with water alone immediately produces a transient increase in the skin pH [source]. Washing the hands with conventional soap causes the pH on the palms to increase by an average of 3 units. Even 90 min after washing with soap the pH of the hands was not completely normalized.[source]
Ok, so not only does water raise the pH of your skin, if you raise the pH of your skin dramatically enough, such as via soap, it will continue to remain raised afterwards. In fact, later I am going to cite a study that shows that there is a cumulative long-term negative effect to spiking the pH of the skin during washing even as infrequently as twice a day.
So, what's so bad about raising the pH of your skin?
Bacteria can grow over a wide pH range, but no microorganisms will grow equally at all pH values [Note: this is important when combined with the information about the 'ideal' range for P. acnes to grow.] Most grow better at a pH around neutrality. Acidic pH could be bacteriostatic [lit: stopping bacteria reproduction without actually killing them] for some strains.
In short, bacteria grows better at a neutral pH, but an acidic pH is less bacteria-friendly. We already know healthy skin is acidic. This becomes very important when it comes to acne, as we're about to see.
The article goes on to cite a study by Korting et al. [source, in full text!] which specifically tested the impact of using soap vs an acidic syndet on inflammatory acne lesions in a 3-month, randomized, open-labeled, comparative trial. Within 4 weeks, the number of lesions on the soap-using group increased, whereas they decreased in acidic syndet using group. Korting et al. flat-out states: "The number of papulopustules characteristic of inflammatory acne thus is clearly lower when a syndet bar of the acidic type is regularly used for cleansing the face as compared to a (necessarily alkaline) soap."
If you're eyes are bleeding, here's the tl;dr: the high pH soap made their acne worse, and the acidic cleanser made it better.
Just take a minute to soak that in. Eye the cleansers in your bathroom. Have you pH tested them? If not, do you know what their pH is? Are you putting acne bait on your face errday?
Now just imagine all those people out there doing DIY baking soda masks and scrubs on their faces. D:
 |
| Preach. Imagine the state of their acid mantle. Shudder. |
Still not convinced there's a link to acne? In Changes in skin pH and resident flora by washing with synthetic detergent preparations at pH 5.5 and 8.5 (full text!) they tested specifically which bacteria were more vs less present while using an acidic (preparation A) vs an alkaline (preparation B) cleanser:
With the propionibacteria [aka P. acnes], however, there was a sharp rise in CFU/ml when the washings with preparation B began, and a sharp fall when the washings with preparation A began. Figures 5 and 6 demonstrate the findings in more detail. At the forehead, propionibacteria were significantly fewer in the presence of the acidic cleansing preparation on day 31.
That's not surprising to me after researching the next point I'm going to discuss. Acidic cleansers don't disrupt the skin's pH like alkaline ones do, and that a low pH is inhospitable to acne (one sources quotes the ideal breeding zone for P. acnes as pH 6.0 to 6.5 but please don't make me go back to figure out which one.)
I've already switched to using just acidic cleansers, with a tearful goodbye to my favourite high-pH foaming cleansers:
Because of their bacteria-regulating properties and favorable tolerability profile, syndets with an acidic pH are now preferred for skin cleansing in patients with seborrheic-type diseases (acne vulgaris, rosacea), atopic skin diathesis, irritant contact dermatitis and ichthyosis. [...] There is good reason to believe that acidic syndets are of value even for persons with healthy skin, for example to prevent rhinovirus transmission and infection.[source]
It still hurts, though. I love(d) my foaming cleansers:
 |
| So long, fabulous bouncy-foam Shiseido Perfect Whip. We had a good run. |
For those of you wondering if having a raised skin pH for just a few hours really matters, let's take a look at The Effect of Detergents on Skin PH and Its Consequences, which goes into more detail about the short-term and long-term negative effects of repeated (short duration) use of alkaline cleansers and even cleansers with a 'neutral' pH of 7.
I found this section to be quite interesting about how even short term exposure impacts the skin over a longer period of time, despite the originally held conclusions on effect duration:
[...] initial data from the 1940s were confirmed in the 1960s. Yet, the effect was considered to be short-lasting: about 2 hours after an individual washing procedure. Given that there are two or three such procedures a day, it seems obvious that there should be no profound effect on related parameters. Against this background it has come as a surprise to many that there are also long-lasting effects with as few as two washing procedures of 1 minute each a day, as we demonstrated at the end of the 1980's. According to a randomized open crossover trial, skin surface pH increases on the regular use of a conventional soap and decreases again after the change to an acidic cleanser (of pH 5.5) and vice versa.
 |
| You can see why this woman is my skin idol. |
As I mentioned in my Skincare Discovery: Cleansing Part II (click) I follow Go Hyun Jung's cleansing method which takes me a hell of a lot longer than 1 minute!
Then later after explaining the use of alkaline, neutral, and acidic cleansers in their tests, and comparing the results:
[...] there is ample evidence that there is both a short-term and long-term effect on skin surface pH if a cleanser is used whose pH deviates from the pH of the skin surface to which it is applied. In keeping with this hypothesis, so-called neutral cleansers are by no means neutral in a biologic sense.
So even 'neutral' cleansers have the short-term and long-term negative effects. As I referenced earlier, that includes water. Anything about pH 5.6, aka the upper range of 'healthy' skin, will raise the skin's pH and cause issues. They finalize with this statement:
Indeed, a large proportion of the general population-- those with polar constitution of the skin surface that is either seborrheic or sebostatic skin-- might profit from the regular use of an acidic cleanser, and there is no reason to believe that it might be disadvantageous in the rest.
The mention of 'seborrheic or sebostatic' threw me off for a minute, but further investigation revealed that seborrheic= oily, and sebostatic = dry. I suppose asking a scientific source to just plainly say "everyone can benefit from an acidic cleanser, oily and dry skin types alike" just doesn't have the same ring to it?
Here's another study that shows acidic pH cleansers scored lower on an Irritation Index than high PH cleansers, and also discusses how repeated exposure has a cumulative effect and can increase the skin's recovery time: Correlation between pH and irritant effect of cleansers marketed for dry skin (full text) which also supports even short, but repeated, exposures does have a cumulative effect.
The claim that alkaline pH = efficacy, is it true?
Lastly, let's circle back around to Oprah's Beauty Expert and her assertion that cleansers need to be alkaline to cleanse properly.
It appears that there is actually no correlation between efficacy of cleansing and higher pH, according to this study: The effect of an acidic cleanser versus soap on the skin pH and micro-flora of adult patients: A non-randomised two group crossover study in an intensive care unit, where they show the low-pH cleanser was just as effective at cleansing as the high-pH cleanser, but with the added benefit of maintaining healthy skin with the acidic cleanser.
Perhaps she is working off the assumption that cleansers need to be soap-based, unaware that syndets are a different animal and can be formulated to let you have the best of both worlds, effective cleansing and a low pH:
Whereas soap has long been the only cleansing agent, a new generation of cleansers, the so-called synthetic detergents or syndets, has been developed during the last decades. [...] Among the syndets, especially those with a pH of about 5.5 seem to be relevant.[source]
However, a quick googling taught me that syndets have been around since 1916, so I'm not sure what's up with that.
How to pH test your products at home?
Ah, that will be for another time. You stare into the pH rabbit hole, the pH rabbit hole stares into you.
Final thoughts:
- Yes, the pH of your cleanser really does matter.
- Your cleanser should be acidic, no more than pH 5.6; anything higher will compromise your skin and worsen acne.
- No, a cleanser doesn't need to be alkaline to be effective. And always do your research. :)
And with that, we're done! If you have a favourite acidic cleanser, please leave me a comment, or connect with me on Facebook or Twitter; I love the Miracle Rose Cleansing Stick but it's so expensive! I need a low-pH alternative, so if you have one, hit me up!
All the best,
-Cat

No comments:
Post a Comment